
For ions that carry the same charge (i.e.Also, effective nuclear charge felt by the outermost electrons decreases. This is because electrons are added to form these ions, increasing electron-electron repulsions, making the electrons spread out more. Anions, or negatively charged ions, are larger than their "parent" atoms.Therefore, the resulting ions are smaller as there are not as many occupied orbitals and the effective nuclear charge affecting the remaining electrons increases, pulling electrons in more closely. This also decreases electron-electron repulsions. This is because cations are formed when those outermost orbitals are vacated of electrons. Cations, or positively charged ions, are smaller than their "parent" atoms.What are the general trends in ionic radii? These distances are based on distances between ions in ionic compounds. Ionic radii are the radii of ions of elements.
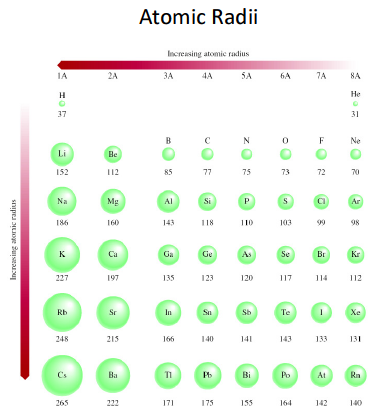

The greater the effective nuclear charge, the more the outer electrons feel that attraction from the nucleus, and the closer those outer electrons are then to the nucleus, making the atom smaller. This is mainly due to effective nuclear charge. Atomic radius generally decreases from left to right across a period.That is, the further down a column, the greater probability of finding the outer electrons further from the atom, making the atom thus larger. This is a result of the increase in the principal quantum number, n. Atomic radius generally increases from top to bottom down a group.What are the general trends in atomic radii? Atomic Radius 1 Arrange the following in order of decreasing atomic radius: Pt, At, Ba, Sm Which element would you predict to have the highest ionization energy Get Document Sailboat Mast Weight Li, Na, K C, N, Si A decreased distance of outer electron b 7 - An ionic compound of potassium and oxygen has the 7 - An ionic compound of potassium. We can then use these values to estimate bond lengths between different elements in molecules. So, if the bond between two Cl atoms in Cl 2 is 1.99 angstroms, we report chlorine's bonding atomic radius as about 0.99 angstroms. These are the radii of atoms that are chemically bonded to one another. Nonbonding atoms have a larger, more undefined or "fuzzy" radius, so when atomic radius is discussed as a periodic trend, what's usually meant is bonding atomic radius. Atomic radii are simply the radii (or half the "width") of these spherical atoms.


 0 kommentar(er)
0 kommentar(er)
